The pharmaceutical industry has slowly and steadily been moving in the direction of continuous manufacturing processes for several years.
As 2020 has so jarringly and intrusively shaken up every aspect of our lives, so too has it altered the way we look at continuous manufacturing, bringing new urgency to the conversation.
Batch processing, while trusted and methodical, is time-consuming. With every step introduced into the process, a new door opens for human error, contamination, and delays. As we’ve seen with the need for fast-tracked COVID-19 vaccines and therapeutics, efficient production is not just a nice-to-have, but can prove to be life-saving on a global scale when it comes to population health and rapid response to medical emergencies.
Vertex’s ORKAMBI® for cystic fibrosis was famously the first to go continuous in 2015, with many others following suit. While continuous manufacturing makes the most sense for new products and new facilities, deviating from batch processing for existing products or facilities can be challenging and costly. The move to continuous is particularly attractive to generics manufacturers looking to increase yield and cut costs.
Let’s explore the pros and cons of batch manufacturing and continuous processing in three critical areas: quality, cost, and safety.
1. Quality
Technology and equipment are now widely available to help achieve continuous and homogenous biopharma production. Continuous mixing processors and bioreactors limit human interaction and thus reduce the likelihood of error and contamination.
The stepwise process of batch manufacturing leaves plenty of opportunities for sterilization and cleaning, while continuous manufacturing requires a different tack. Specially designed cleaning procedures, fluid management systems, sterilization pumps, and inline process analytic technologies can help reduce contamination risk while keeping product cycles continuous.
In-process monitoring devices, smart sensors, machine learning tools, and artificial intelligence enable smart manufacturing and provide process analytics without breaching a bioreactor’s sterility.
The qualification and monitoring of quality management systems are critical to avoiding quality issues and related costs.
2. Cost
One study estimates that inefficient batch processes cost the pharmaceutical industry upwards of $50 billion annually due to contamination, losses, and recalls.
The National Science and Technology Council also reported that a 40–50% reduction in variable costs is achievable with continuous manufacturing, which could be instrumental in making drugs more affordable to patients and increasing the availability of critical pharmaceuticals, both major topics of conversation in the U.S.
While the overhead costs to get started with continuous manufacturing are not insignificant, consider the efficiencies and savings associated with continuous manufacturing:
- Time savings and shorter production cycles
- Reduced energy consumption
- Increased productivity
- Less waste
- Reduced risk of human error
- Higher quality and fewer recalls
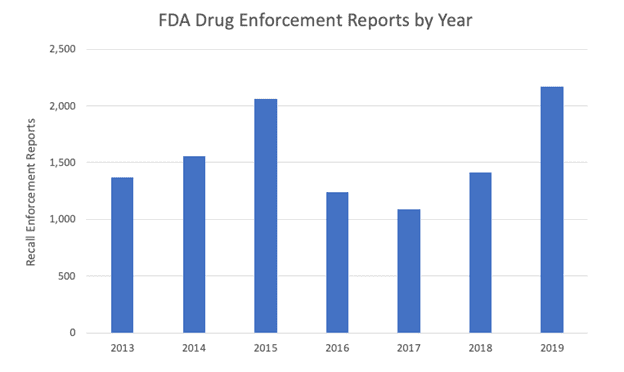
With recalls proving to be the costliest quality issue for manufacturers, recall avoidance is key. Safety and reliability in the manufacturing process are critical to reversing the trend of rising FDA drug enforcement reports, as seen below.
3. Safety
Continuous processing offers significant risk mitigation, mainly due to increased control and less handling of hazardous materials. The conditions inside the processing equipment remain stable and the potential for harmful reactions is much lower with less human intervention.
In this COVID world we live in today, automation plays a critical role in reducing the number of people required to operate equipment, helping to maintain social distancing requirements and conserve personal protective equipment.
Where Do You Stand on Continuous vs. Batch Manufacturing?
Our team has extensive knowledge of the commissioning, qualification, and validation of equipment and processes involved in continuous manufacturing and has executed various projects for continuous manufacturing plants.
If you’re considering switching to continuous processing or want to learn more about the CQV implications, contact us to have a conversation.
Whether you’re utilizing batch manufacturing, continuous, or a combination of both, we can ensure your processes are as efficient, safe, and reproducible as possible.