Stainless Steel vs. Single-Use Systems: Cost and Time Savings
Stainless steel has long been the preferred material biologics manufacturing equipment. However, single-use systems (SUS) have continued to grow in popularity since the mid-2000s due to their potential for economic savings, smaller footprint, and increased efficiency.
Disposable technologies offer the most benefit to smaller companies working with minimal funding to reduce operating costs and minimize environmental impact. In a heavily regulated industry, SUS and disposable technologies offer flexibility in researching, developing, manufacturing, and marketing drug products.
While SUS only accounts for approximately 10% of total industry capacity, the proportion jumps to 25 to 50% for new installations.
Here we discuss the many benefits of SUS and disposable technologies.
Time and Cost Savings with Single-Use Systems
Reduced Cleaning Costs and Carbon Footprint
Biopharmaceutical manufacturers are increasingly conscious of their impact on the environment. Recent data has shown that utilizing SUS decreases energy, water, chemical, electrical, and even heating and air conditioning usage.
SUS and disposable technologies clearly do not require the intensive cleaning and sanitation processes that stainless steel demands in addition to cleaning validation, which amounts to significant time and resource savings. SUS come pre-sterilized and production-ready, which eliminates clean in place (CIP) and steam in place (SIP) processes — saving money, time, water, chemicals, and complex plumbing components.
Due to their reduced physical footprint, utilizing SUS can also help to downsize facility square footage requirements. Companies with less capital can operate smaller facilities for early development and clinical trials with reduced costs related to facility size and utility consumption.
Time Savings
The reduction in cleaning, sterilization, and validation requirements also allows for quicker setup of new processing lines with smaller footprint facilities and lower construction costs. Not only will biomanufacturing companies realize greater efficiency, but they will also have more opportunities to institute flexible manufacturing facilities.
Although SUS bioprocessing requires continual equipment expenditures to replace systems that have been used to their capacity, the cost and time savings related to avoided cleaning, sterilization, and validation — along with the gained facility flexibility — almost always outweigh the repeated technology costs.
Water and Utility Consumption Savings
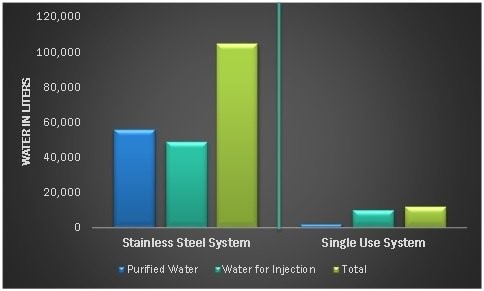
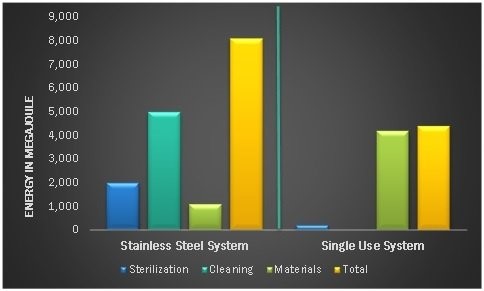
Elimination of cleaning and sterilization processes comes with significant utility savings in water and energy. With no pre- or post-processing cleaning, SUS saves roughly 86% of water consumption and 80% of energy consumption compared to stainless steel facilities.
Figure 1. Water Consumption: Stainless Steel Systems vs. Single-Use Systems. Source: Bioprocess International
Figure 2. Energy Consumption: Stainless Steel Systems vs. Single-Use Systems. Source: Bioprocess International
Saving with Single-Use Systems
The time savings with SUS allow biopharma companies to build capacity at a later stage in the clinical cycle where there is more certainty that a therapeutic will reach the market. Capacity can also be expanded in phases as demand increases.
With estimated reductions in capital expenditures of up to 50% and decreases in water and energy consumption of up to 80% compared to traditional stainless steel facilities, single-use manufacturing technologies can help companies increase capacity more quickly and repurpose capital savings toward other projects for growth and expansion, including pipeline development.
Get It Done Right: Contact ICQ to Get Started
Want to discuss the implications of single-use systems on biopharma commissioning, qualification, and validation? Get in touch with our CQV experts today.